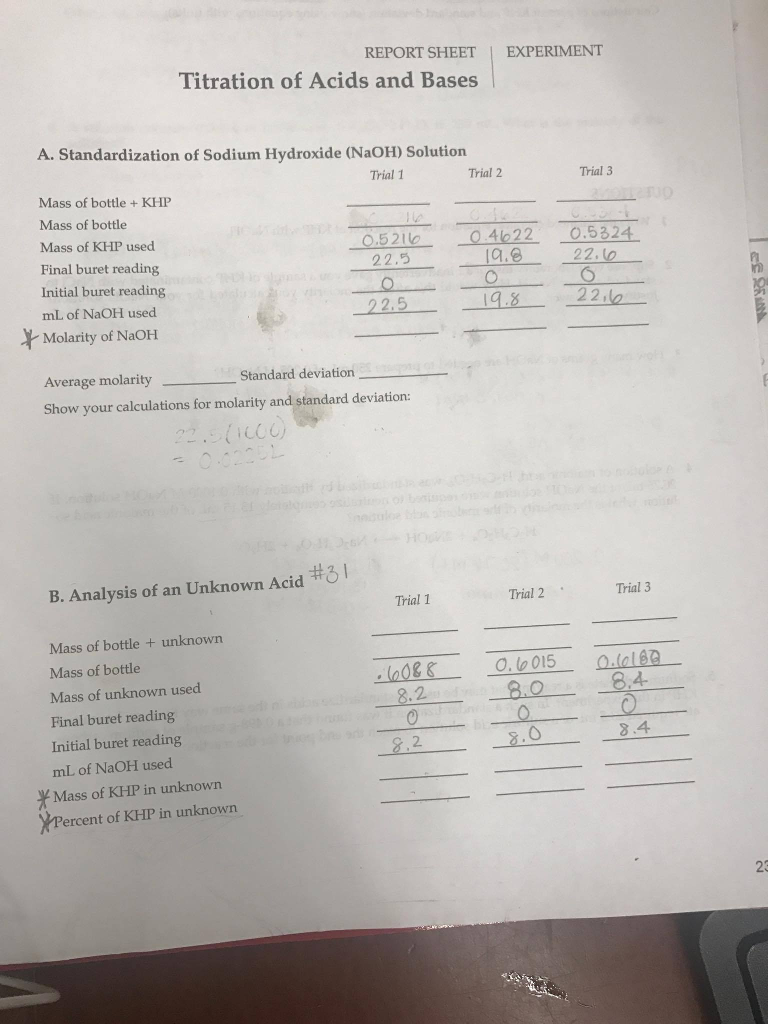
6 Complete Approaches For Reacting Khp With Naoh For Maximum Efficiency

The reaction between potassium hydrogen phthalate (KHP) and sodium hydroxide (NaOH) is a commonly used titration method in analytical chemistry for determining the concentration of NaOH solutions. This reaction is highly efficient due to the strong acid-base interaction between KHP and NaOH. To maximize efficiency, several approaches can be employed, focusing on precision, safety, and environmental considerations. Here, we delve into six complete approaches for reacting KHP with NaOH for maximum efficiency.
Understanding the Reaction Basics
The chemical reaction between KHP and NaOH can be represented by the equation: KHC8H4O4 + NaOH → NaKC8H4O4 + H2O. This equation shows a 1:1 mole ratio between KHP and NaOH, making it straightforward to calculate the concentration of NaOH based on the amount of KHP used and the volume of NaOH required to reach the equivalence point. Accurate measurement of both reactants is crucial for the success of the titration. The stoichiometry of the reaction dictates that for every mole of KHP, one mole of NaOH is required to neutralize it completely.
Preparation of KHP and NaOH Solutions
Preparation of the solutions is a critical step. KHP is typically used as a primary standard because it is highly pure and stable, making it ideal for precise titrations. NaOH solutions, on the other hand, can absorb carbon dioxide from the air, which affects their concentration. Therefore, NaOH solutions should be freshly prepared or standardized before use. The preparation involves dissolving a known amount of KHP in water to create a solution of known concentration, and for NaOH, it involves dissolving NaOH pellets in water, followed by standardization using KHP or another strong acid.
| Substance | Molecular Weight | Concentration |
|---|---|---|
| KHP (KC8H5O4) | 204.22 g/mol | 0.1 M |
| NaOH | 40.00 g/mol | To be determined |

Titration Techniques for Maximum Efficiency

Titration techniques play a significant role in the efficiency of the reaction. Potentiometric titration and colorimetric titration are common methods. Potentiometric titration involves measuring the change in electrode potential as the reaction proceeds, offering high precision. Colorimetric titration, which uses an indicator to signal the equivalence point, is simpler but requires careful selection of the indicator to ensure it changes color at the correct pH.
Automation and Precision
Automated titrators can significantly enhance the precision and efficiency of the titration process. These devices can accurately dispense the titrant (NaOH) and detect the endpoint with high sensitivity, reducing human error. Additionally, they can perform multiple titrations in a row, making them ideal for laboratories with a high workload.
For manual titrations, using a buret with a sharp tip and a magnetic stirrer can improve the accuracy of the titration by ensuring thorough mixing and precise measurement of the NaOH added.
Data Analysis and Interpretation
After completing the titration, the data collected needs to be analyzed to determine the concentration of the NaOH solution. This involves calculating the amount of NaOH used to reach the equivalence point and relating it to the known amount of KHP. Graphical analysis can also be employed, especially in potentiometric titrations, to visualize the titration curve and more accurately determine the equivalence point.
Futures Implications and Environmental Considerations
The reaction between KHP and NaOH, while highly useful, also has environmental implications. The disposal of chemical waste from such reactions must be handled responsibly to prevent environmental harm. Future research may focus on developing more environmentally friendly methods for titration or exploring alternative reactants that are less harmful to the environment.
What is the significance of using KHP as a primary standard in titrations?
+KHP is used as a primary standard because it is highly pure, stable, and its molecular weight is well-defined, making it ideal for precise titrations and concentration calculations.
How does the presence of CO2 affect NaOH solutions?
+CO2 reacts with NaOH to form sodium carbonate, which decreases the concentration of NaOH in the solution. This can lead to inaccurate titration results if not accounted for.
In conclusion, the reaction between KHP and NaOH is a fundamental process in analytical chemistry that can be optimized through careful preparation, precise measurement, and the use of automated titration techniques. By understanding the stoichiometry of the reaction, employing accurate measurement techniques, and considering environmental implications, laboratories can achieve maximum efficiency in their titration processes.


