How Can Hydrogen Bonding Distance Be Used To Predict Chemical Reaction Outcomes Accurately
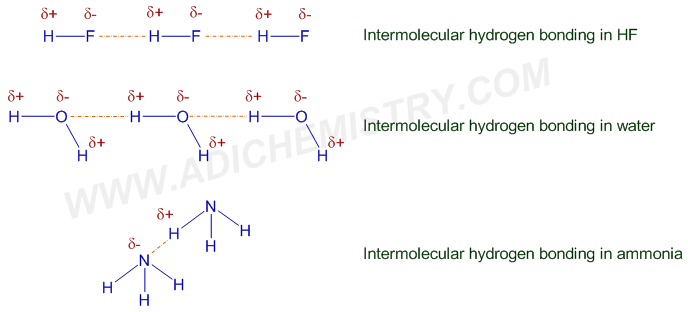
Hydrogen bonding is a crucial aspect of chemistry, playing a significant role in the structure and function of molecules. The distance between molecules involved in hydrogen bonding can be a critical factor in determining the outcome of chemical reactions. By understanding and accurately measuring hydrogen bonding distances, chemists can gain valuable insights into the behavior of molecules and predict the outcomes of chemical reactions with greater precision. In this context, the accurate measurement of hydrogen bonding distances can be a powerful tool for predicting chemical reaction outcomes.
Introduction to Hydrogen Bonding and Its Importance in Chemical Reactions
Hydrogen bonding is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This interaction is characterized by a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom, resulting in an attractive force between the molecules. Hydrogen bonding is essential in many biological and chemical processes, including protein folding, DNA replication, and chemical catalysis. The strength and nature of hydrogen bonding can significantly influence the outcome of chemical reactions, making it crucial to understand and predict these interactions accurately.
Methods for Measuring Hydrogen Bonding Distances
Several experimental and computational methods can be employed to measure hydrogen bonding distances, including X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and molecular dynamics simulations. X-ray crystallography provides high-resolution structural information, allowing for the precise determination of hydrogen bonding distances in crystalline solids. NMR spectroscopy, on the other hand, offers insights into the dynamic behavior of molecules in solution, enabling the measurement of hydrogen bonding distances in a more flexible and realistic environment. Molecular dynamics simulations, based on ab initio calculations or force fields, can also be used to predict hydrogen bonding distances and simulate the behavior of molecules under various conditions.
| Method | Description | Advantages |
|---|---|---|
| X-ray Crystallography | High-resolution structural determination | High accuracy, precise distance measurements |
| NMR Spectroscopy | Dynamical behavior of molecules in solution | Insights into molecular flexibility, solvent effects |
| Molecular Dynamics Simulations | Predictive modeling of molecular behavior | Flexibility, scalability, simulation of various conditions |
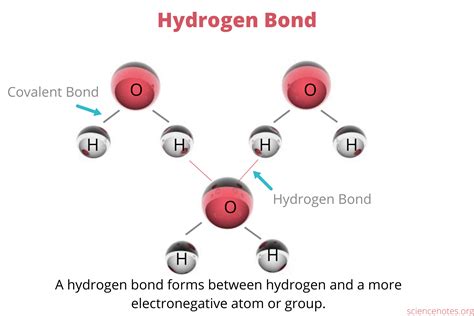
Prediction of Chemical Reaction Outcomes Based on Hydrogen Bonding Distances

By accurately measuring and understanding hydrogen bonding distances, chemists can predict the outcomes of chemical reactions with greater precision. Hydrogen bonding distances can influence reaction rates, selectivity, and yields, as they affect the stability and reactivity of molecules. For instance, shorter hydrogen bonding distances can indicate stronger interactions, potentially leading to increased reaction rates or altered product distributions. Conversely, longer distances may result in weaker interactions, affecting the reaction mechanism or outcome. The analysis of hydrogen bonding distances can also reveal insights into the role of solvents, catalysts, or other reaction conditions, enabling the optimization of reaction conditions for improved efficiency and selectivity.
Case Studies and Examples
Several case studies demonstrate the importance of hydrogen bonding distances in predicting chemical reaction outcomes. For example, the enzymatic catalysis of peptide bond formation involves specific hydrogen bonding interactions between the enzyme, substrate, and solvent molecules. Understanding these interactions and their associated distances can provide insights into the reaction mechanism and guide the design of more efficient catalysts. Similarly, the self-assembly of supramolecular structures relies on precise control over hydrogen bonding distances, which can be achieved through careful design of the molecular components and reaction conditions.
- Enzymatic catalysis of peptide bond formation: understanding hydrogen bonding distances can guide catalyst design and optimization
- Self-assembly of supramolecular structures: precise control over hydrogen bonding distances enables the creation of complex architectures
- Chemical synthesis: hydrogen bonding distances can influence reaction rates, selectivity, and yields, affecting the overall efficiency and outcome of the reaction
What is the significance of hydrogen bonding distances in predicting chemical reaction outcomes?
+Hydrogen bonding distances can influence reaction rates, selectivity, and yields, as they affect the stability and reactivity of molecules. Accurate measurement and understanding of these distances can provide valuable insights into the behavior of molecules and predict the outcomes of chemical reactions with greater precision.
How can hydrogen bonding distances be measured and analyzed?
+Hydrogen bonding distances can be measured using various experimental and computational methods, including X-ray crystallography, NMR spectroscopy, and molecular dynamics simulations. These methods provide insights into the structural and dynamical properties of molecules, enabling the analysis of hydrogen bonding distances and their role in chemical reactions.
In conclusion, the accurate measurement and understanding of hydrogen bonding distances can be a powerful tool for predicting chemical reaction outcomes. By considering the specific distances and interactions involved in hydrogen bonding, chemists can gain valuable insights into the behavior of molecules and optimize reaction conditions for improved efficiency and selectivity. As research continues to advance in this field, the development of more sophisticated methods for measuring and analyzing hydrogen bonding distances will likely play a crucial role in shaping our understanding of chemical reactions and the design of new catalysts, materials, and technologies.