How Often Should I Review The Capability Index Formula For Quality Assurance 2025?
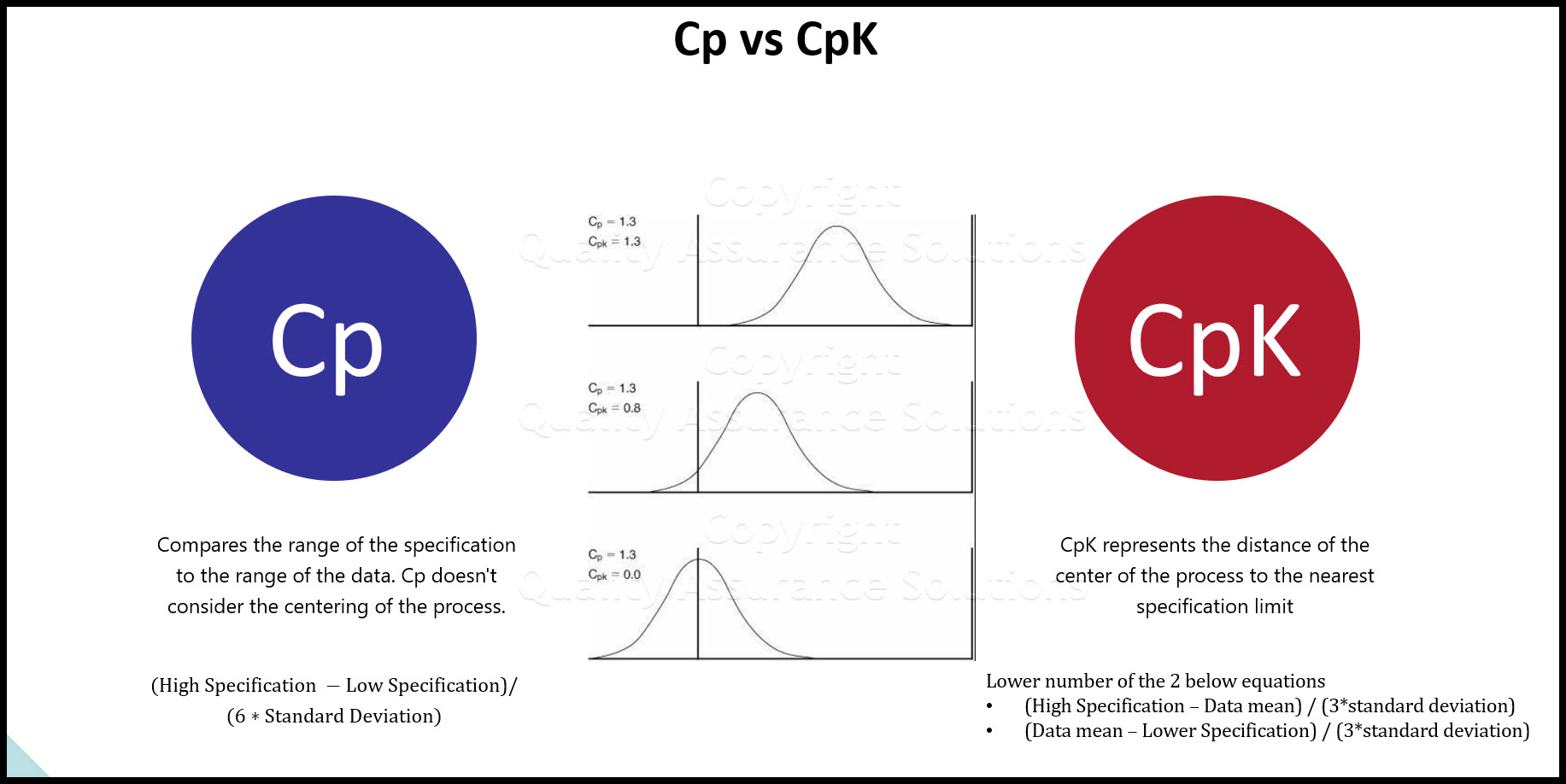
The Capability Index Formula, also known as the Cpk (Capability Index), is a statistical measure used in quality assurance to determine the ability of a process to produce output within specified limits. Reviewing this formula is crucial for maintaining high-quality standards in manufacturing and production processes. The frequency of reviewing the Capability Index Formula depends on various factors, including the type of industry, process stability, and the level of quality control required.
Understanding the Capability Index Formula

The Capability Index Formula is calculated using the following equation: Cpk = min(Cpu, Cpl), where Cpu = (USL - μ) / (3σ) and Cpl = (μ - LSL) / (3σ). Here, USL is the upper specification limit, LSL is the lower specification limit, μ is the process mean, and σ is the standard deviation of the process. This formula provides a quantitative measure of the process capability, helping quality assurance teams to identify areas for improvement.
Factors Influencing Review Frequency
The review frequency of the Capability Index Formula can be influenced by several factors, including:
- Process Stability: If the process is stable and the capability index is consistently high, the review frequency can be lower. However, if the process is unstable or the capability index is low, more frequent reviews may be necessary.
- Industry Requirements: Certain industries, such as aerospace or healthcare, may require more frequent reviews due to stringent quality control requirements.
- Quality Control Requirements: The level of quality control required can also influence the review frequency. For example, if the process is critical to product safety, more frequent reviews may be necessary.
| Industry | Review Frequency |
|---|---|
| Aerospace | Every 3-6 months |
| Healthcare | Every 6-12 months |
| Automotive | Every 12-18 months |

Best Practices for Reviewing the Capability Index Formula

When reviewing the Capability Index Formula, it is essential to follow best practices, including:
- Use Historical Data: Use historical data to calculate the capability index and identify trends or patterns.
- Monitor Process Changes: Monitor process changes and recalculate the capability index as needed.
- Involve Cross-Functional Teams: Involve cross-functional teams, including quality assurance, engineering, and production, to ensure that everyone is aware of the process capability and any necessary changes.
Future Implications
The Capability Index Formula will continue to play a critical role in quality assurance in 2025 and beyond. As industries become increasingly complex and quality control requirements become more stringent, the need for regular reviews of the Capability Index Formula will become even more essential. By following best practices and reviewing the formula regularly, organizations can ensure that their processes remain capable of producing high-quality products, reducing waste, and improving customer satisfaction.
What is the purpose of the Capability Index Formula?
+The purpose of the Capability Index Formula is to determine the ability of a process to produce output within specified limits, ensuring high-quality products and reducing waste.
How often should I review the Capability Index Formula?
+The review frequency of the Capability Index Formula depends on various factors, including process stability, industry requirements, and quality control requirements. It is essential to review the formula regularly to ensure that the process remains capable of producing output within specified limits.
