How To Improve Understanding Of Nickel Heat Capacity For 2025 Academic Purposes
Nickel, a ferromagnetic transition metal, has been a subject of interest in various fields, including physics, chemistry, and materials science. Its unique properties, such as high thermal conductivity and resistance to corrosion, make it an essential material in numerous applications. For academic purposes in 2025, understanding nickel's heat capacity is crucial, as it plays a significant role in determining the metal's thermal behavior. In this context, heat capacity refers to the amount of heat energy required to change the temperature of a substance by a given amount. This article aims to provide a comprehensive overview of nickel's heat capacity, its significance, and methods to improve understanding of this property for academic purposes.
Introduction to Nickel Heat Capacity

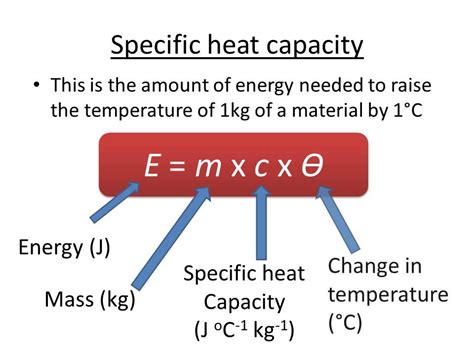
Nickel’s heat capacity is a critical parameter in understanding its thermal properties. The heat capacity of nickel is typically expressed in units of joules per gram per kelvin (J/g·K) or joules per mole per kelvin (J/mol·K). At room temperature, the heat capacity of nickel is approximately 0.444 J/g·K. This value indicates that nickel requires about 0.444 joules of heat energy to raise its temperature by 1 Kelvin. The heat capacity of nickel is influenced by various factors, including temperature, pressure, and the presence of impurities. Therefore, understanding these factors is essential for accurate calculations and applications.
Factors Influencing Nickel Heat Capacity
Several factors can affect the heat capacity of nickel, including:
- Temperature: Nickel’s heat capacity varies with temperature. At higher temperatures, the heat capacity increases due to the increased vibrational motion of the atoms.
- Pressure: Changes in pressure can also impact nickel’s heat capacity. High pressures can lead to a decrease in heat capacity due to the reduced interatomic spacing.
- Impurities: The presence of impurities, such as carbon or oxygen, can alter nickel’s heat capacity. These impurities can introduce additional vibrational modes, affecting the overall heat capacity.
Understanding these factors is crucial for accurately predicting and measuring nickel’s heat capacity. Thermodynamic models, such as the Debye model, can be employed to estimate the heat capacity of nickel based on its temperature and pressure dependence.
Methods to Improve Understanding of Nickel Heat Capacity

To improve understanding of nickel’s heat capacity for academic purposes in 2025, several methods can be employed:
- Experimental Measurements: Direct measurements of nickel’s heat capacity can be performed using techniques such as differential scanning calorimetry (DSC) or adiabatic calorimetry. These methods involve measuring the heat flow into or out of a sample as its temperature is changed.
- Theoretical Modeling: Computational models, such as density functional theory (DFT) or molecular dynamics simulations, can be used to predict nickel’s heat capacity based on its atomic structure and interactions.
- Literature Review: A comprehensive review of existing literature on nickel’s heat capacity can provide valuable insights into its temperature and pressure dependence, as well as the effects of impurities.
By combining these methods, researchers and students can gain a deeper understanding of nickel’s heat capacity and its significance in various applications.
| Temperature (K) | Heat Capacity (J/g·K) |
|---|---|
| 300 | 0.444 |
| 500 | 0.513 |
| 800 | 0.623 |

This table illustrates the temperature dependence of nickel’s heat capacity, demonstrating an increase in heat capacity with temperature.
Applications of Nickel Heat Capacity

Nickel’s heat capacity has numerous applications in various fields, including:
- Aerospace Engineering: Nickel alloys are used in aircraft and spacecraft due to their high thermal conductivity and resistance to corrosion. Understanding nickel’s heat capacity is essential for designing efficient cooling systems.
- Energy Storage: Nickel is used in thermal energy storage systems, such as molten salt batteries, due to its high heat capacity and thermal conductivity.
- Chemical Processing: Nickel’s heat capacity is important in chemical processing, particularly in catalytic reactions, where temperature control is crucial.
These applications highlight the significance of nickel’s heat capacity in various industries and the need for accurate understanding and measurement of this property.
What is the significance of nickel's heat capacity in aerospace engineering?
+Nickel's heat capacity is crucial in aerospace engineering for designing efficient cooling systems, particularly in aircraft and spacecraft. The high thermal conductivity and heat capacity of nickel alloys enable the effective dissipation of heat, ensuring the structural integrity and performance of these systems.
How can experimental measurements improve our understanding of nickel's heat capacity?
+Experimental measurements, such as differential scanning calorimetry (DSC) or adiabatic calorimetry, can provide direct and accurate measurements of nickel's heat capacity. These techniques involve measuring the heat flow into or out of a sample as its temperature is changed, allowing researchers to determine the heat capacity of nickel with high precision.
In conclusion, understanding nickel’s heat capacity is essential for various academic and industrial applications. By employing experimental measurements, theoretical modeling, and literature reviews, researchers and students can gain a deeper understanding of this property and its significance in different fields. The applications of nickel’s heat capacity in aerospace engineering, energy storage, and chemical processing highlight the importance of accurate measurements and predictions of this property.



