How To Solve Specific Heat Of Magnesium Problems Step By Step In 1 Hour
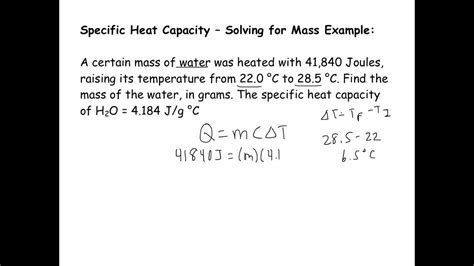
The specific heat of magnesium is a fundamental concept in physics and chemistry, representing the amount of heat energy required to raise the temperature of a unit mass of magnesium by one degree Celsius. Solving problems related to the specific heat of magnesium involves understanding the formula and applying it correctly to given scenarios. In this guide, we will walk through the steps to solve specific heat of magnesium problems efficiently within a short timeframe, such as one hour.
Understanding the Specific Heat Formula
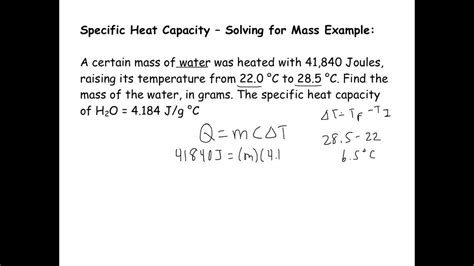
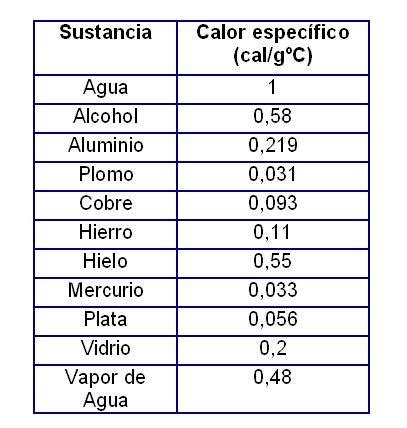
The formula for specific heat is Q = mcΔT, where Q is the amount of heat added to or removed from the substance, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature. The specific heat of magnesium is approximately 1.02 J/g°C. To solve problems, one must identify the given values and the unknown variable that needs to be calculated.
Step-by-Step Solution Approach
To solve specific heat problems of magnesium within a short time frame like one hour, follow these steps:
- Read the Problem Carefully: Understand what is given and what needs to be found. Identify the mass of magnesium, the initial and final temperatures, and any other relevant information.
- Apply the Specific Heat Formula: Use the formula Q = mcΔT and rearrange it to solve for the unknown variable. For example, if you need to find the mass (m) of magnesium, rearrange the formula to m = Q / (cΔT).
- Perform Calculations: Substitute the given values into the rearranged formula and calculate the unknown variable. Ensure that all units are consistent; for instance, if the specific heat is given in J/g°C, the mass should be in grams, and the temperature change in degrees Celsius.
- Check Units and Significant Figures: Verify that the units of the calculated value are appropriate for the problem. Also, ensure that the answer is reported with the correct number of significant figures based on the given data.
Example: A piece of magnesium with a mass of 50 grams is heated from 20°C to 100°C. How much heat is required? Given that the specific heat of magnesium is 1.02 J/g°C, we can calculate the heat required as follows:
Q = mcΔT = 50 g * 1.02 J/g°C * (100°C - 20°C) = 50 * 1.02 * 80 = 4080 J
| Given Values | Units |
|---|---|
| Mass of Magnesium | 50 g |
| Initial Temperature | 20°C |
| Final Temperature | 100°C |
| Specific Heat of Magnesium | 1.02 J/g°C |
| Calculated Heat | 4080 J |

Common Challenges and Solutions

One common challenge is ensuring that units are consistent throughout the calculation. Another challenge is correctly applying the formula to solve for the unknown variable. To overcome these, always double-check the units of the given values and the formula used for calculation.
Tips for Quick Problem Solving
To solve specific heat problems of magnesium quickly and accurately:
- Ensure a thorough understanding of the specific heat formula and its application.
- Practice solving different types of problems to enhance speed and accuracy.
- Always verify the units of the given values and the calculated answer.
- Use a systematic approach to solving problems, following the steps outlined above.
What is the specific heat of magnesium and how is it used in calculations?
+The specific heat of magnesium is approximately 1.02 J/g°C. It is used in the formula Q = mcΔT to calculate the heat required to change the temperature of a given mass of magnesium by a certain amount.
How do I ensure accurate calculations when solving specific heat problems?
+Accurate calculations can be ensured by verifying the units of the given values, applying the correct formula, and performing calculations carefully. It's also important to check the significant figures of the calculated answer based on the given data.
By following the steps and tips outlined in this guide, and practicing with various problems, one can efficiently solve specific heat of magnesium problems within a short time frame like one hour. Remember, the key to quick and accurate problem-solving is a thorough understanding of the concept, careful application of the formula, and attention to detail in calculations.