How To Understand Helium Specific Heat Properties For Advanced Engineering Designs
Helium, as the second most abundant element in the universe, has unique properties that make it an essential component in various advanced engineering applications, including cryogenics, superconductivity, and aerospace engineering. One of the critical properties of helium is its specific heat capacity, which is crucial in understanding its behavior under different conditions. The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of a unit mass of the substance by one degree Celsius. In the case of helium, its specific heat properties are vital for designing efficient and safe systems that utilize this gas.
Helium has two stable isotopes, helium-4 and helium-3, each with distinct specific heat properties. Helium-4, being the more abundant isotope, has a specific heat capacity at constant volume (Cv) of approximately 3/2 R, where R is the gas constant, and at constant pressure (Cp) of about 5/2 R. These values are theoretical and based on the ideal gas model. However, real-world applications often involve non-ideal gas behavior, especially at low temperatures or high pressures, necessitating more complex equations of state to accurately predict helium's specific heat properties.
Understanding Specific Heat Capacity of Helium
The specific heat capacity of helium, like any other gas, can be described in terms of its molecular structure and the degrees of freedom available to its molecules. For monatomic gases like helium, the molecules can move in three dimensions, contributing to their translational kinetic energy. According to the equipartition theorem, each degree of freedom contributes 1/2 R to the specific heat capacity at constant volume. Therefore, for an ideal monatomic gas, Cv = 3/2 R, and since the specific heat at constant pressure (Cp) includes the work done in expanding the gas, Cp = Cv + R = 5/2 R.
However, helium exhibits quantum behavior at very low temperatures, leading to deviations from classical ideal gas behavior. Below a certain critical temperature (around 4.2 K for helium-4), helium becomes a superfluid, with properties that significantly differ from its behavior above this temperature. In the superfluid state, helium can exhibit zero viscosity and other unique properties that are crucial for certain applications, such as cooling superconducting materials or magnets.
Factors Influencing Helium's Specific Heat Properties
Several factors can influence the specific heat properties of helium, including temperature, pressure, and the presence of impurities. At high temperatures, helium behaves more like an ideal gas, with its specific heat capacity approaching the theoretical values. However, at lower temperatures, especially near its critical point or in the superfluid regime, helium's behavior becomes more complex, and its specific heat capacity can deviate significantly from ideal gas predictions.
Pressure is another critical factor, as high pressures can cause helium to deviate from ideal gas behavior, even at room temperature. This deviation is due to the increased density of the gas, which leads to interactions between molecules that are not accounted for in the ideal gas model. In engineering applications, understanding these deviations is crucial for designing systems that operate efficiently and safely under a wide range of conditions.
| Property | Helium-4 | Helium-3 |
|---|---|---|
| Atomic Mass | 4.0026 u | 3.0160 u |
| Critical Temperature | 5.2 K | 3.3 K |
| Specific Heat at Constant Volume (Cv) | 3/2 R | 3/2 R |
| Specific Heat at Constant Pressure (Cp) | 5/2 R | 5/2 R |
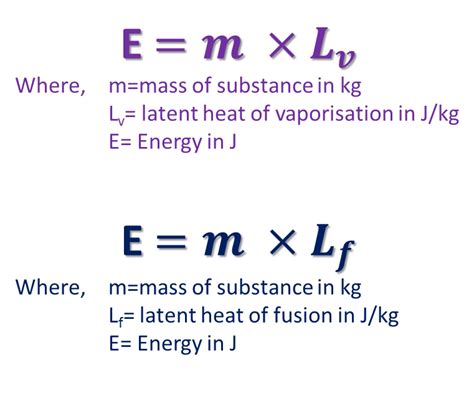
The application of helium's specific heat properties is seen in various fields, including the cooling of superconducting materials, where maintaining temperatures below the critical temperature is essential for superconductivity. In aerospace engineering, helium is used as a pressurant gas for rocket fuel tanks due to its low molecular weight and high specific heat capacity, which helps in maintaining the tank's internal pressure and temperature during ascent.
Advanced Engineering Applications

One of the most significant applications of helium's specific heat properties is in the field of superconductivity. Superconducting materials have zero electrical resistance when cooled below their critical temperature, making them ideal for applications such as magnetic resonance imaging (MRI) machines, high-energy particle accelerators, and power transmission lines. Helium, particularly helium-4, is used as a coolant because it can be cooled to very low temperatures (below 4.2 K) where it becomes superfluid, providing highly efficient heat transfer.
In cryogenic engineering, the specific heat properties of helium are critical for the design of cryogenic storage tanks, heat exchangers, and other equipment. The ability to predict and manage the thermal behavior of helium under various conditions is essential for the safe and efficient operation of these systems. Furthermore, the use of helium in cryogenic applications has led to the development of advanced materials and technologies, such as superconducting magnets and cryogenic pumps.
Cryogenic Applications and Safety Considerations
Cryogenic applications of helium require careful consideration of safety due to the risks associated with extremely low temperatures and high pressures. The handling of liquid helium, for instance, demands specialized equipment and training to prevent accidents. Moreover, the design of cryogenic systems must account for the thermal expansion and contraction of materials, as well as the potential for cryogenic liquids to rapidly expand if they are inadvertently released.
Despite these challenges, the unique properties of helium make it an indispensable component in many advanced engineering applications. Its specific heat capacity, combined with its low molecular weight and chemical inertness, makes it an ideal gas for cooling, pressurization, and other purposes. As research and development continue to push the boundaries of what is possible with helium, understanding its specific heat properties will remain a critical aspect of designing and operating efficient, safe, and innovative systems.
What is the significance of helium's specific heat capacity in cryogenic applications?
+Helium's specific heat capacity is crucial in cryogenic applications because it determines how much heat energy is required to change the temperature of the gas. This property is essential for the efficient cooling of superconducting materials and the design of cryogenic systems.
How does the superfluid state of helium affect its specific heat properties?
+In the superfluid state, helium exhibits unique properties, including zero viscosity and high thermal conductivity, which significantly affect its specific heat capacity. The superfluid state allows for highly efficient heat transfer, making it ideal for cooling applications.
What safety considerations are critical when handling helium in cryogenic applications?
+Handling helium in cryogenic applications requires careful consideration of safety due to the risks associated with extremely low temperatures and high pressures. Specialized equipment, training, and protocols are necessary to prevent accidents and ensure safe operation.
In conclusion, understanding helium’s specific heat properties is fundamental for the design and operation of advanced engineering systems, particularly those involving cryogenic temperatures or high pressures. The unique properties of helium, including its superfluid state, offer unparalleled opportunities for innovation in fields such as superconductivity, aerospace, and materials science. As research continues to explore the boundaries of helium’s applications, the importance of its specific heat properties will only continue to grow.
