Hydrogen Bonding Distance
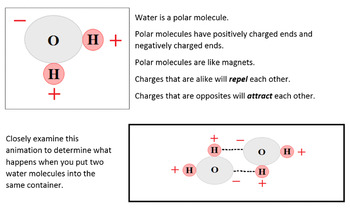
Hydrogen bonding is a fundamental concept in chemistry, playing a crucial role in the structure and properties of molecules. It is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The distance between the hydrogen atom and the electronegative atom is a critical factor in determining the strength and stability of hydrogen bonds. In this context, understanding the hydrogen bonding distance is essential for elucidating the mechanisms of various chemical and biological processes.
Definition and Characteristics of Hydrogen Bonding Distance
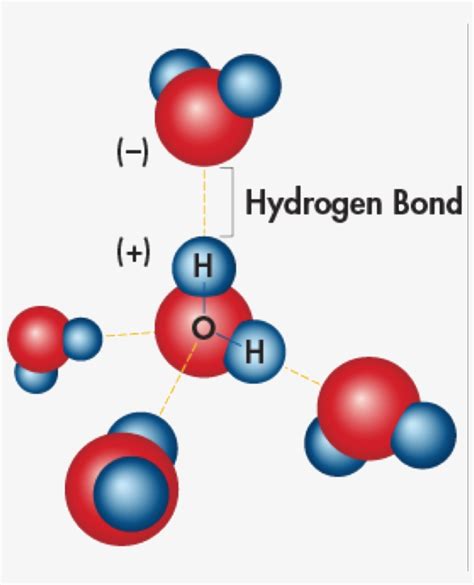
The hydrogen bonding distance, also known as the hydrogen bond length, refers to the distance between the hydrogen atom and the electronegative atom involved in the hydrogen bond. This distance is typically measured in angstroms (Å) or picometers (pm). The strength of a hydrogen bond is inversely proportional to the distance between the hydrogen atom and the electronegative atom. In general, shorter hydrogen bonding distances result in stronger hydrogen bonds, while longer distances lead to weaker bonds. The characteristics of hydrogen bonding distances can be influenced by various factors, including the type of electronegative atom, the molecular environment, and the presence of other intermolecular forces.
Factors Influencing Hydrogen Bonding Distance
Several factors can influence the hydrogen bonding distance, including the electronegativity of the atom bonded to the hydrogen, the size and shape of the molecules involved, and the presence of other intermolecular forces. For example, electronegativity plays a crucial role in determining the hydrogen bonding distance, as more electronegative atoms tend to form stronger hydrogen bonds with shorter distances. Additionally, the steric effects of large molecules can influence the hydrogen bonding distance by limiting the accessibility of the hydrogen atom to the electronegative atom. Furthermore, the presence of other intermolecular forces, such as van der Waals forces or ionic interactions, can also affect the hydrogen bonding distance.
| Type of Hydrogen Bond | Hydrogen Bonding Distance (Å) |
|---|---|
| O-H...O | 1.5-2.5 |
| N-H...O | 1.7-2.7 |
| N-H...N | 1.9-3.0 |
| O-H...N | 1.8-2.8 |
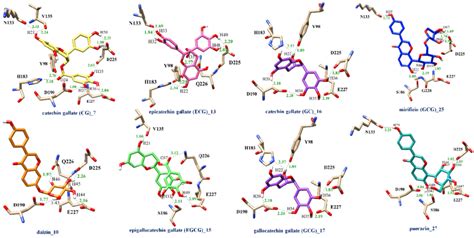
Measurement and Analysis of Hydrogen Bonding Distance

The measurement and analysis of hydrogen bonding distances are essential for understanding the mechanisms of various chemical and biological processes. Several experimental techniques, such as X-ray crystallography and neutron diffraction, can be used to determine the hydrogen bonding distances in crystals and other solids. Additionally, computational methods, such as molecular mechanics and quantum mechanics, can be employed to predict the hydrogen bonding distances in molecules and complexes. The analysis of hydrogen bonding distances can provide valuable insights into the strength and stability of hydrogen bonds, as well as the mechanisms of various chemical and biological processes.
Applications of Hydrogen Bonding Distance Analysis
The analysis of hydrogen bonding distances has numerous applications in chemistry, biology, and materials science. For example, understanding the hydrogen bonding distances in proteins and nucleic acids is essential for elucidating the mechanisms of protein folding and DNA replication. Additionally, the analysis of hydrogen bonding distances in supramolecular complexes can provide valuable insights into the design and synthesis of new materials with unique properties. Furthermore, the study of hydrogen bonding distances in liquids and solutions can help to understand the mechanisms of various chemical reactions and biological processes.
- Protein folding and stability
- DNA replication and transcription
- Supramolecular complexation and self-assembly
- Liquid and solution chemistry
What is the typical range of hydrogen bonding distances?
+The typical range of hydrogen bonding distances is between 1.5 and 3.0 Å, depending on the type of hydrogen bond and the molecular environment.
How do electronegativity and steric effects influence hydrogen bonding distances?
+Electronegativity and steric effects can influence hydrogen bonding distances by affecting the accessibility of the hydrogen atom to the electronegative atom and the strength of the hydrogen bond.
In conclusion, the hydrogen bonding distance is a critical factor in determining the strength and stability of hydrogen bonds, which play a crucial role in various chemical and biological processes. Understanding the factors that influence hydrogen bonding distances, such as electronegativity and steric effects, is essential for predicting the strength and stability of hydrogen bonds. The measurement and analysis of hydrogen bonding distances using experimental and computational techniques can provide valuable insights into the mechanisms of various chemical and biological processes, with numerous applications in chemistry, biology, and materials science.

